Summary
What is a mixture and a solution

A bowl of cereal shows dried fruits, oats, wheat, etc. This bowl represents a mixture of several things or substances. A mixture consists of two or more different substances that are mixed. You can separate mixtures by physical means.
Let’s look at the following pictures. Can you determine the components of the following mixtures?
Mixtures can be separated. Sometimes you may want to separate a mixture. Some mixtures are very easy to separate. You can separate the mixtures above. They can be hand-picked.

Hand-picking is a simple method to separate substances. It involves the manual use of your hand to pick out a substance from a mixture. Do you think is is easy to hand-pick some nails that is mixed in a container of corn.
You should also note that there are mixtures where hand-picking is not practical. Such mixtures can also be sieved. A sieve has small pores that allow smaller particles to pass through them and retain the larger particles. Do you think a sieve would work to separate the mixture below. How about separating rice and flour? Do you think we can separate rice and flour?

A solution can be solids dissolved in liquids. Scientist say a solution is a group of molecules that are mixed and evenly distributed (spread out) in a system (referred to as a homogeneous system). A mixture that has a higher concentration of one thing is referred to as heterogeneous mixtures.
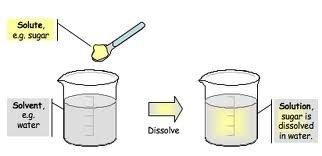
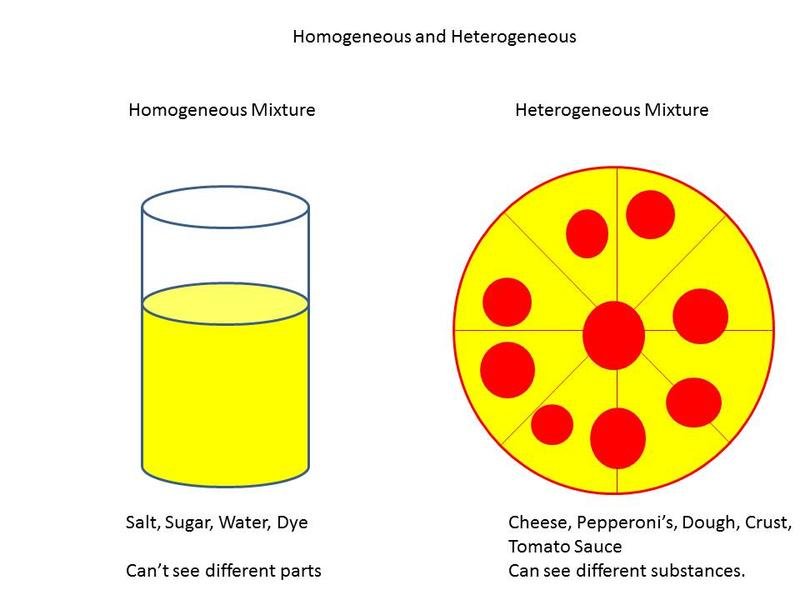
Any mixture that stay at an even distribution is a solution.
Thus, many things are mixtures. Everything around you is a mixture, such as the air you breath, rocks, etc. Mixtures are all about its physical properties.

Exercise: Making a solution.

Many times it may be necessary to separate mixtures.
Separating Mixtures
Separation of two solids will depend on the nature of the solid. Mixtures come in many forms and separation depends on the kind of mixture. Here are some common methods of separation.
Winnowing
Winnowing is a process to separate mixtures by using the wind. A good example is the separation of husk from grain.
Hand picking
Hand picking is a process of separating components manually and is based on the principle of shape, size or colour of the components. One of the components is present in a small quantity because it is identifiable. Stones in pulses can be separated. This method is time consuming.

separation by Magnetism
Mixtures of two solids can be separated, one have magnetic properties, by a process called magnetism. The process involves the use of a magnet. Solids that have magnetic properties are iron, nickel and cobalt. Gold, silver and aluminum are not magnetic.
Exercise:

separation by filtration
Some mixtures can be separated with the help of a strainer or sieve. Such mixtures contains one insoluble solid and the mixture is sieved through a porous material. The larger particles are separated. When you boil cocoa tea, you use the method of filtration to remove particles of cocoa from the tea.
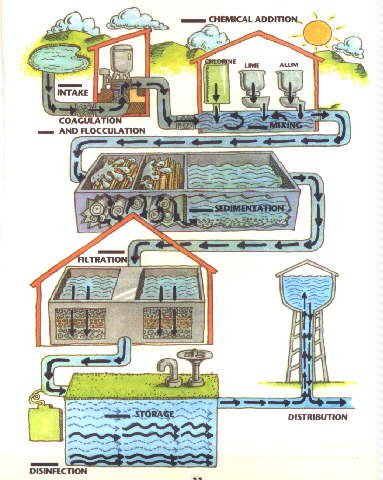
Filtration is used in water treatment plants.
Below is a diagram that shows the water treatment process.

Separation by distillation
Distillation helps separate a substance from a liquid mixture by boiling or condensation.
A simple distillation is used if you want to separate water from salt.
Chromatography
Chromatography is a technique of separation used in chemical laboratories. Chromatography is a technique of separating two or more dissolved solids which are present in a solution in very small quantities.
Worksheets
Click on the links below to download the PDF worksheets (exercises and questions).